By Nathan Smith, doctoral candidate in biochemistry and biophysics
Let’s talk about Alzheimer’s disease. Alzheimer’s is a neurodegenerative disease, which means the disease is characterized by loss of brain density or a loss of brain cells. The brain cells die over time. Alzheimer’s disease affects six million Americans. That means it affects more than three times as many people in this country than all forms of cancer combined. So why aren’t we talking about it more?
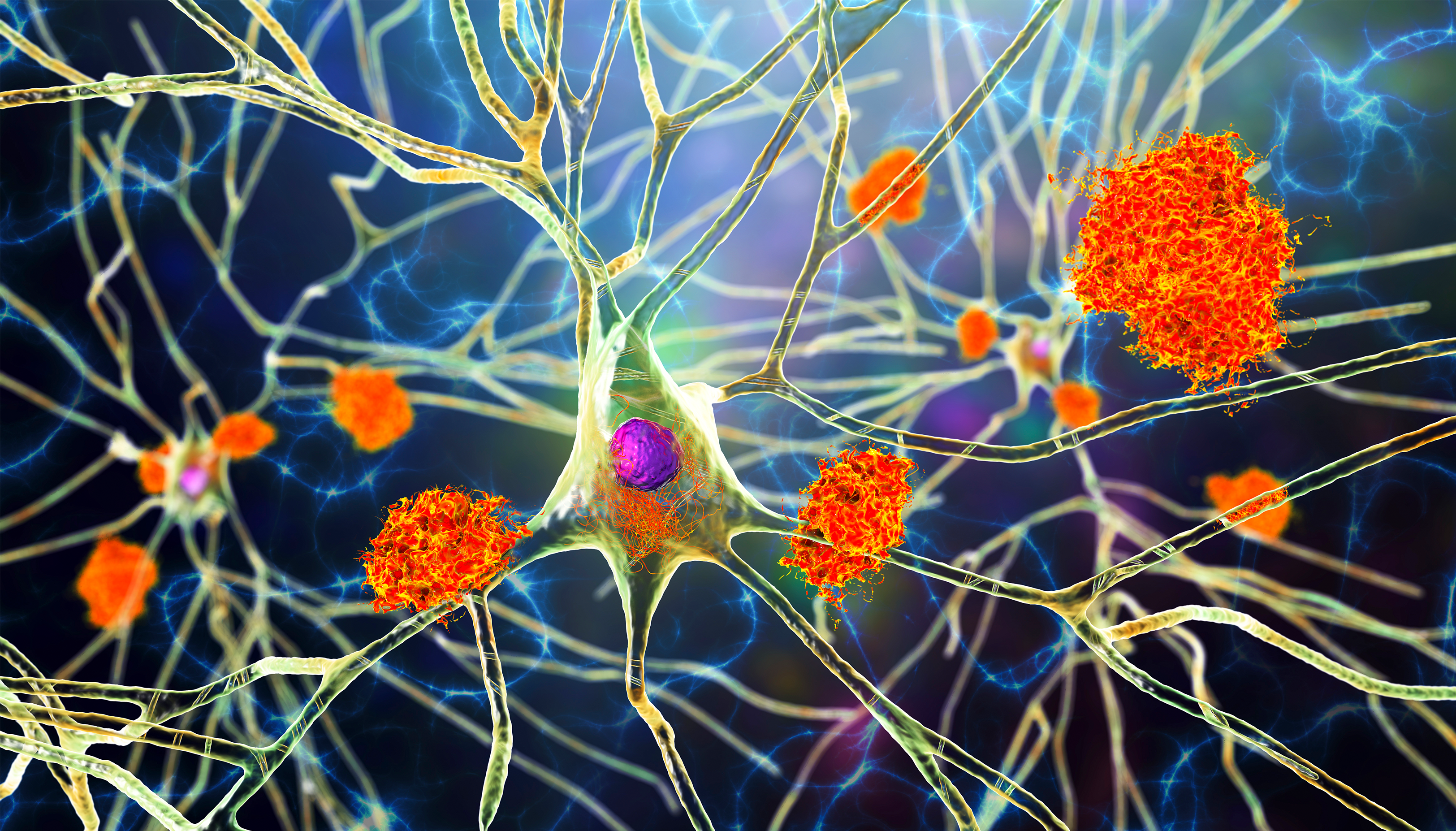
To begin, we must talk about protein. When I say protein, I don’t mean protein powder or milk or cheese or meat or whatever you might think of first when you hear protein. What I’m talking about is amyloid beta and tau. Tau forms bundles called neurofibrillary tangles and amyloid beta forms plaques.
When you hear plaque, you might think about the plaque on your teeth or the plaque in your arteries, which isn’t too far off. The plaque in your arteries prevents the transport of blood throughout your body and could ultimately lead to cell death or a heart attack. The same thing is true in your brain. When these plaques build up in your brain cells, it prevents accurate and efficient transport. It prevents your brain cells from getting things to where they need to be, and ultimately leads to brain cell death. And when your brain cells die, your brain starts to shrink.
Alzheimer’s patients begin to experience shrinking of the brain, atrophy, or the death of brain cells over time, which eventually leads to the loss of speech, the loss of memory, and the overall loss of independence.
The scary thought is that there is no cure for Alzheimer’s. Right now, there are very few efficient treatments either. So, our lab has been trying to work with a new compound called carbon dots.
Carbon dots were discovered in 2004 when researchers were trying to build carbon nanotubes, very thin carbon and nitrogen dense tubes used for transport. But in the process of being built, they began to degrade and some broke down into dust. When the researchers looked at the particles, they discovered a new, unique compound called carbon dots.
In the 20 years since, we’ve used them for a range of different purposes, from dyes to stains to drug delivery, and most importantly, for drug development. We’ve been trying to use these carbon dots to turn back the clock and return proteins to their original state.
Amyloid beta and tau aren’t normally bad for us. We need these proteins to function normally. But when they aggregate, when they bundle together, that’s when they’re toxic. So, we want to return them to this healthy, normal state.
To examine this, we use a fluorescent dye called Thioflavin T. We put this dye into a solution with the proteins to tell us if bundles are present. If they’re present, we can conclude that the aggregation is still taking place. If we use different recipes for carbon dots and different concentrations, we can try to reduce this fluorescent signal.
If we can successfully return proteins to a normal, healthy state, it offers not only new hope to patients to gain their speech and their independence back, but also a new hope for curing Alzheimer’s disease.
The content of this post, which has been edited for clarity, was originally part of RPI’s Three Minute Thesis Competition, which challenges doctoral students to effectively explain their research to a general audience in three minutes.